Hypothesis: Could Native Hypoxic LV Ejection Drive Arrhythmias in Peripheral VA ECMO?
Key Points
Right radial ABGs may not reflect coronary oxygenation in peripheral VA ECMO.
Hypoxic native LV ejection can perfuse the coronaries, even when ECMO support looks adequate.
This may contribute to recurrent VT/VF or ectopy that doesn't respond to shocks or medications.
Devices like Impella and low ventilator FiO₂ settings may unintentionally worsen the problem.
Adjusting FiO₂ or using early V-VA ECMO could improve coronary oxygenation without surgical escalation.
Introduction
This is a working hypothesis I’ve been thinking through as I try to better understand what we see during peripheral VA ECMO. I’m still early in my ECMO learning and always trying to make sense of how physiology plays out in real patients.
One question that keeps coming up for me is this:
Could the heart be perfusing itself with hypoxic blood, even when the right radial ABG looks perfectly fine?
This article walks through that idea and considers whether differential hypoxemia, something we already recognize in peripheral VA ECMO, might be playing a bigger role in arrhythmias than we typically assume, especially when native LV output is still present.
Understanding the Potential Problem: Peripheral VA ECMO Dynamics
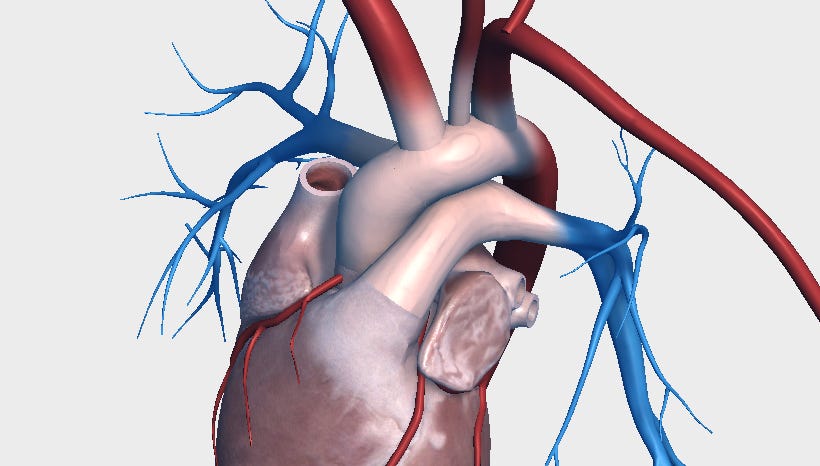
Right radial arterial blood gases are frequently used as a surrogate for upper body oxygenation and are often assumed to reflect coronary perfusion. However, anatomically, that assumption may not hold.
The coronary arteries branch directly off the aortic root, just two to three centimeters above the aortic valve. The brachiocephalic artery, which supplies the right radial artery, originates farther up in the aortic arch, approximately four to five centimeters from the valve.
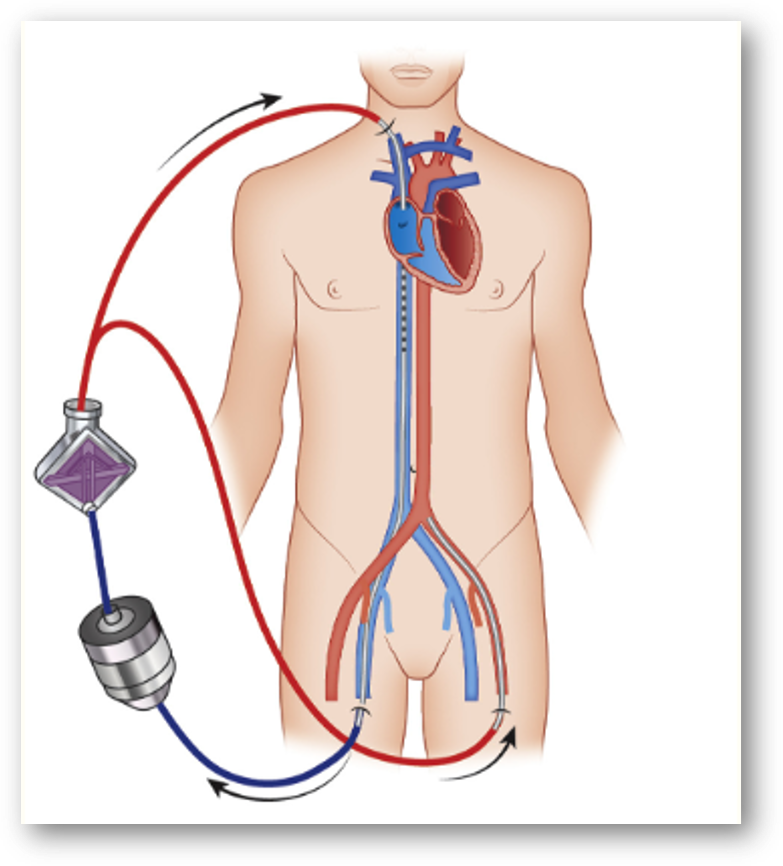
In peripheral VA ECMO (e.g., femoral-femoral cannulation), oxygenated blood from the ECMO circuit flows retrograde up the aorta, while the patient’s native heart ejects blood antegrade. If pulmonary function is significantly impaired, this native ejection may consist of hypoxic blood.
These two flow streams meet at a constantly moving “mixing point” in the aorta. The location of this point fluctuates based on the strength of native cardiac output and ECMO flow rates. This anatomical difference is well understood, but its clinical implication, especially in relation to coronary perfusion, is often underappreciated.
The Danger Zone Hypothesis: Mixing Above the Coronaries
This hypothesis centers on a particular scenario: If the patient’s native cardiac output, even if weak, ejects hypoxic blood that reaches as far as the coronary arteries, but not as far as the brachiocephalic artery, then the following may occur:
The potentially deceptive right radial ABG: If the mixing point lies distal to the brachiocephalic takeoff, the right radial artery may receive fully oxygenated ECMO blood, and an ABG from that site could appear reassuring.
The hidden threat: If mixing occurs between the coronary ostia and the brachiocephalic artery, the myocardium may be perfused with hypoxic blood from the native circulation, while the right radial ABG falsely suggests adequate systemic oxygenation.
In this scenario, recurrent ectopy, VT, or VF may not simply reflect electrical irritability but rather may represent the heart’s physiologic response to ischemia.
Connecting the Hypothesis to Arrhythmias and Ectopy
Myocardial tissue is highly sensitive to oxygen deprivation. If the coronary arteries are intermittently or persistently perfused with hypoxic blood:
Myocardial ischemia develops.
Electrical instability follows, increasing the risk of ventricular arrhythmias (VT/VF) or frequent ectopy (e.g., PVCs).
The hypothesis suggests that when clinicians observe refractory arrhythmias or worsening ectopy, especially when paired with labile or inconsistent right radial oxygenation, they should consider whether unseen coronary hypoxia may be driving the instability.
Treating the Rhythm, Missing the Cause
This leads to a familiar and frustrating clinical picture: A defibrillator at the bedside, the crash cart parked just outside the room, and the patient cycling in and out of VT/VF despite sedation and escalating antiarrhythmic therapy. Lidocaine, amiodarone, magnesium, and even ganglion blocks are attempted, yet the arrhythmias persist.
If the myocardium is ischemic due to hypoxic native LV ejection, these arrhythmias may stem directly from coronary under-oxygenation. In that case, focusing solely on electrical suppression may fail to resolve the underlying problem. Without addressing the oxygen content of blood perfusing the coronaries, arrhythmias may continue regardless of drug therapy or shocks.
When Support Strategies Worsen Coronary Hypoxia
Mechanical support devices such as Impella are often used during peripheral VA ECMO to reduce left ventricular distention and support forward flow. While beneficial for pressure unloading, these devices do not address the oxygen content of blood entering the left ventricle.
In fact, Impella may deliver hypoxic blood from the LV directly into the proximal aorta, where the coronary arteries arise. If the lungs are underperforming and ventilator FiO₂ is low, this native blood may remain desaturated, and coronary perfusion could be compromised despite good right radial oxygenation.
Potential Solutions
Central Cannulation
Escalating to central ECMO can effectively resolve this issue by ensuring antegrade delivery of oxygenated blood to the coronary arteries. However, this is an invasive step, typically reserved for patients in severe decline, and not always feasible in a timely manner.
Early V-VA ECMO Configuration
Rather than increasing ECMO flow, which can worsen LV distention, or waiting until central cannulation becomes necessary, V-VA ECMO may offer an intermediate solution. In this configuration, a portion of oxygenated blood from the arterial limb is returned to the venous drainage limb, enhancing the oxygen content of blood passing through the lungs and ultimately into the LV.
Benefits of this approach may include:
Improved venous oxygenation, even in low-functioning lungs
More oxygenated blood entering the left atrium and ventricle
Reduced hypoxia in native LV ejection
Better coronary perfusion, potentially breaking the ischemia-arrhythmia cycle
Reconsidering Ventilator Management and the Broader Risk of Coronary Hypoxia
In peripheral VA ECMO, ventilators are often set to “rest settings,” low respiratory rate, low tidal volume, and low FiO₂, based on the assumption that the ECMO circuit provides adequate systemic oxygenation. These settings help minimize ventilator-induced lung injury and reduce pulmonary oxygen toxicity, especially when arterial PaO₂ levels (such as in the right radial) are already elevated due to the ECMO flow.
However, when any native pulmonary blood flow persists, as is often the case, even modestly hypoxic blood may pass through the lungs, enter the left atrium and ventricle, and be ejected by the native heart. If this ejected blood is under-oxygenated and reaches the coronary arteries, it may contribute to myocardial ischemia, even if global oxygen delivery seems sufficient and the right radial ABG appears reassuring.
Increasing ventilator FiO₂, even if it does not significantly affect the right radial PaO₂, may still improve the oxygen content of this small but critical volume of blood. That improvement could meaningfully impact coronary perfusion, particularly in patients showing new ectopy or ventricular arrhythmias.
Importantly, this issue is not limited to patients with severely diseased lungs. Even when pulmonary function is relatively preserved, ventilator FiO₂ may be kept low to avoid oxygen-related lung injury. Yet in the presence of even minor ventilation-perfusion mismatch, this can result in desaturated pulmonary venous blood. That blood, if ejected by the LV, may perfuse the coronaries with insufficient oxygen, despite an apparently normal or even hyperoxic right radial ABG.
This expands the relevance of the hypothesis beyond classic ARDS physiology and highlights the importance of asking not just “what does the ABG say?” but:
“What is the myocardium actually receiving?”
Conclusion
This remains a physiologic hypothesis I have been thinking about: Early V-VA ECMO configurations, along with thoughtful ventilator management (especially FiO₂ adjustments), might improve coronary oxygenation in select patients on peripheral VA ECMO. In cases of recurrent arrhythmias, we may need to ask whether the myocardium is being perfused with hypoxic blood, even when conventional markers like the right radial ABG appear normal.
I am still relatively new to ECMO and learning every day. It is possible that what feels like a major realization to me may already be well understood by others. But sometimes, as the saying goes, "Actual learning requires that you do those things." (Frank Herbert)
Clinicians managing refractory arrhythmias in pVA ECMO may benefit from widening their diagnostic lens and considering interventions that specifically improve myocardial oxygen delivery, rather than relying solely on rhythm suppression.
Note
This article is for educational purposes and is not a substitute for professional medical advice. Always consult qualified healthcare professionals for patient care and clinical decisions.
Subscribe to ECMO 143
If you found this helpful, follow my free LinkedIn newsletter, ECMO 143: AI-Assisted Journey, where I share my ongoing learning in ECMO, physiology, and critical care.
My ECMO Projects and Tools
I’ve built the following platforms to support both clinical professionals and families navigating ECMO:
lifesupport.training – My primary platform for full-length ECMO articles, future ELSO exam preparation, and certification support (ACLS/PALS/BLS/ATLS)
ecmo.life – A simplified ECMO explainer site for patients and families
AI Tools I created and actively use the following custom GPTs to support my research and writing process:
AI ECMO Expert – A physiologic reasoning assistant trained to analyze ECMO-specific documents
Micro Definitions (MD-GPT) – A terminology tool to clarify clinical terms in real time
Thanks to OpenEvidence, GPT-4o, Perplexity, Gemini Advanced 2.5 Pro, Claude 3.7, Grammarly, Leonardo AI, DALL·E 3, Microsoft Designer, and others.
Image Credit
Visual Anatomy 3D – Human, by GraphicVizion. Used with permission.
References
Extracorporeal Life Support Organization (ELSO). ELSO Red Book: Guidelines for Cardiopulmonary Extracorporeal Life Support Organization. 5th ed. ELSO, 2017.
Lorusso R, Shekar K, MacLaren G, Schmidt M, Pellegrino V, Fraser JF, eds. ECMO Specialist Training Manual. 1st ed. Extracorporeal Life Support Organization; 2018.