As a new ECMO Specialist, I navigate through unfamiliar territories, encountering situations that challenge my knowledge and understanding. One such scenario that has aroused my curiosity is the simultaneous use of an Intra-Aortic Balloon Pump (IABP) with Bi-Fem Peripheral Veno-Arterial (VA) ECMO. While these two lifesaving interventions are often employed in critical care settings, the intricacies of their combined application have raised several questions that I aim to address through this post.
The decision to use an IABP with Bi-Fem Peripheral VA ECMO is complex. It requires careful consideration of the patient’s clinical condition, hemodynamic status, and the combined therapy’s potential benefits and risks. Here are some common questions that arise when faced with this scenario:
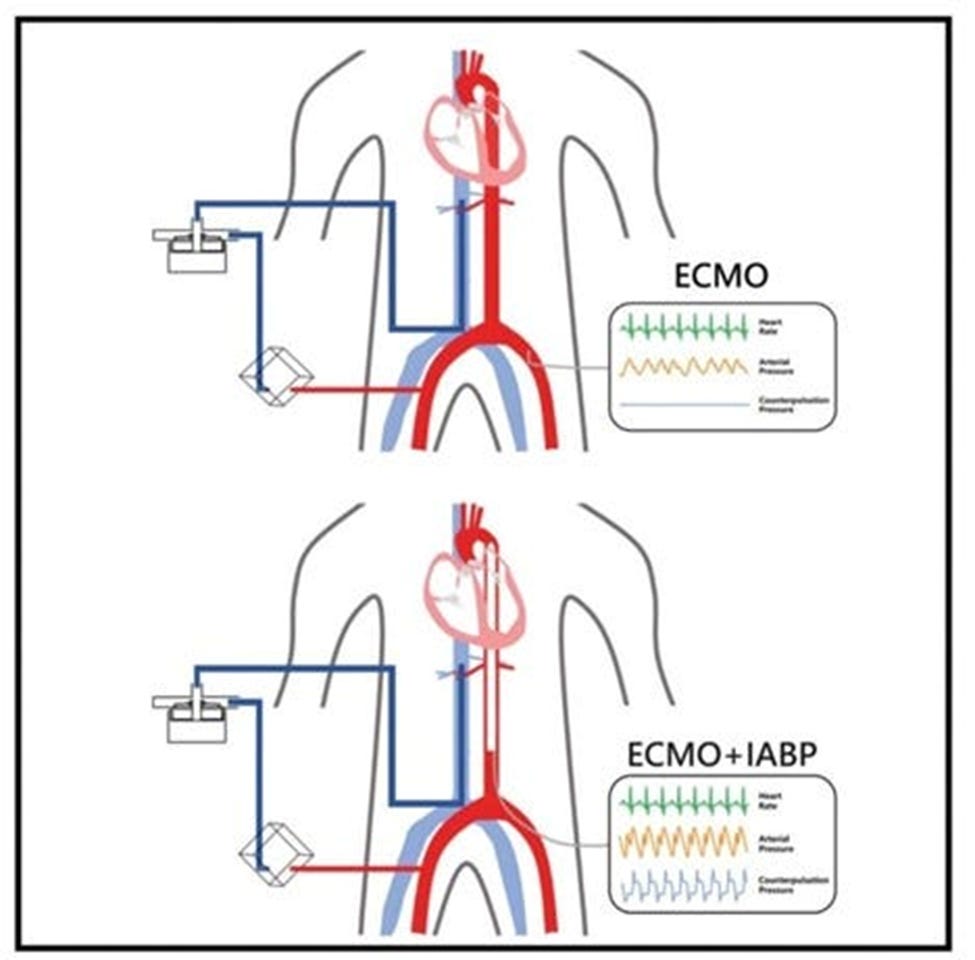
1. THE BIGGEST QUESTION: Why Use VA ECMO and IABP together?
As a novice exploring the intricate world of ECMO, I’ve come to appreciate the combined use of Veno-Arterial Extracorporeal Membrane Oxygenation (VA ECMO) and Intra-Aortic Balloon Pump (IABP) in complex cardiovascular support cases. When used together, these technologies target scenarios that enhance each other’s effectiveness, producing outcomes not achievable with either intervention alone. Here are some of the instances:
a. Enhanced Hemodynamic Management in Cardiogenic Shock: I’ve learned that patients with severe cardiogenic shock, especially following acute myocardial infarction, often require more than just mechanical circulatory or respiratory support. While VA ECMO ensures vital organ perfusion, it can inadvertently increase left ventricular afterload, adding stress to an already failing heart. The IABP counters this by reducing the afterload and improving myocardial oxygen delivery. Together, they stabilize a patient’s hemodynamics, improving survival chances until more definitive interventions can be performed.
b. Prevention of Ventricular Distension: I’ve learned that using VA ECMO alone, especially in patients with compromised left ventricular function, risks left ventricular distension, which can lead to pulmonary edema and increased thrombogenesis (the formation of blood clots within blood vessels). IABP alleviates this by enhancing myocardial perfusion and assisting left ventricular ejection, preventing the adverse effects of blood stasis and pressure overload.
c. Optimized Bridge to Recovery or Transplant: In cases where patients await heart transplantation, keeping optimal cardiac and systemic conditions is crucial. While VA ECMO provides the necessary life support, it doesn’t effectively address myocardial ischemia or ventricular workload on its own. Adding IABP can enhance myocardial perfusion and reduce workload, making it an ideal adjunct to ECMO by potentially improving the heart’s condition before transplant or allowing it to recover sufficiently to prevent the need for transplant.
d. Management of Post-Cardiotomy Cardiogenic Shock: The post-cardiotomy period, particularly after complex and lengthy cardiac surgeries, can lead to significant myocardial stunning and low cardiac output syndrome. While VA ECMO supports these patients by ensuring oxygenation and circulation, adding an IABP specifically helps recover cardiac function by reducing myocardial oxygen demand and improving coronary blood flow, which is crucial in the immediate postoperative period.
2. How does the IABP work, and what are its primary functions?
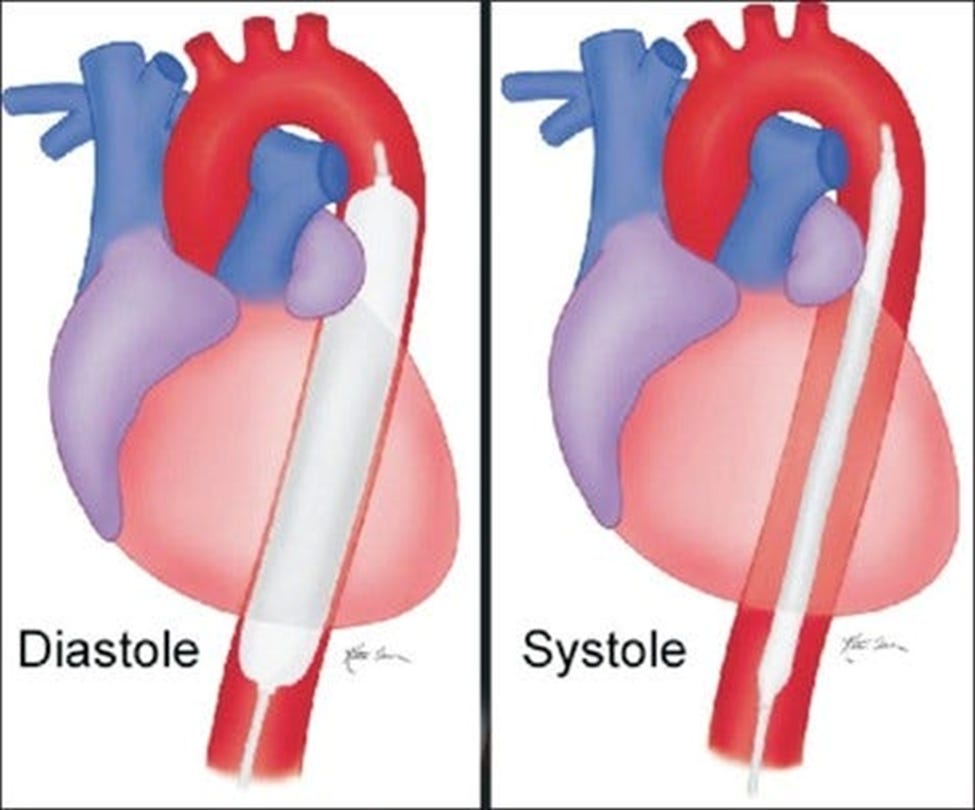
a. How does it work? The IABP is a mechanical device that consists of a balloon catheter inserted into the aorta, typically via the femoral artery. During diastole (the heart’s relaxation phase), the balloon inflates, increasing aortic pressure and improving coronary perfusion. This is crucial for patients with compromised cardiac function, as it enhances blood flow to the heart muscle, potentially preventing or mitigating myocardial ischemia. Conversely, during systole, the heart’s contraction phase, the balloon deflates rapidly. This deflation reduces the aortic pressure, decreasing the left ventricular afterload—the pressure against which the heart must pump blood. This mechanical reduction in afterload is beneficial as it reduces myocardial oxygen demand and improves cardiac output, thereby enhancing the overall hemodynamic performance of the heart.
b. Benefits: The primary goal of the IABP is to optimize myocardial oxygen balance by simultaneously increasing oxygen supply and reducing oxygen demand. This dual action is particularly beneficial in scenarios of acute coronary syndrome or heart failure where myocardial oxygen demand exceeds supply. By improving myocardial oxygen delivery and reducing workload, the IABP can stabilize critically ill patients and enhance myocardial recovery post-cardiac surgery.
3. If a patient is on an IABP and peripheral Bi-Fem VA ECMO, where are the feasible placement sites, and do the two devices conflict?
a. Placement: The IABP catheter is typically inserted into the femoral artery, while the ECMO circuit involves cannulating both femoral vessels (artery and vein).
b. Working together: While the concurrent placement of these devices may seem conflicting, they can coexist if proper precautions are taken to prevent kinking or obstruction of the vasculature. Careful positioning and securing the catheters and cannulas are essential to ensure adequate blood flow and prevent complications.
c. Considerations: Additionally, the femoral artery used for IABP placement may need to differ from the one used for ECMO cannulation to avoid interference and potential limb ischemia.
4. What are the potential complications of using both an IABP and BiFem Peripheral VA ECMO at the same time?
a. Vascular Complications: Limb Ischemia from reduced blood flow distal to the cannulation sites due to large-bore vascular cannulation. Vascular Injury: Risks include arterial dissection, hematoma, or pseudoaneurysm.
b. Bleeding and Hematologic Issues: Bleeding is increased due to necessary anticoagulation and potential vascular trauma. Thrombocytopenia and Coagulopathies: Both devices can worsen coagulopathies by affecting platelet function.
c. Infection Risks: Cannulation Site Infections due to prolonged cannulation heightens the risk of infections, especially in critically ill patients.
d. Hemodynamic Challenges: Increased afterload from ECMO can worsen left ventricular function, countering the afterload reduction aimed at IABP. ECMO can complicate the timing of IABP balloon inflation/deflation, affecting its effectiveness.
e. Management Complexity: Effective management requires intensive monitoring, substantial resources, and a skilled multidisciplinary team. When using both devices, careful monitoring, vigilant hemodynamic assessment, and coordinated care are crucial to addressing complications effectively.
5. Is it advisable to remove one of the devices (IABP or ECMO) to simplify the management?
a. Decision Making: The decision to discontinue the IABP or ECMO should be based on a comprehensive evaluation of the patient’s clinical status, hemodynamic parameters, and the potential risks and benefits associated with each intervention.
b. IABP Removal: The IABP may be removed if the patient’s hemodynamic status improves and the ECMO support is sufficient to maintain adequate perfusion. However, if the IABP provides significant hemodynamic support and improves coronary perfusion, its removal may compromise the patient’s condition.
c. ECMO Removal: Conversely, discontinuing ECMO may not be advisable if the IABP provides sufficient hemodynamic support and the patient’s condition is still critical. ECMO provides respiratory and circulatory support, and its removal could lead to life-threatening complications.
6. If both ECMO and the IABP are in use, is it better to trigger the IABP with pressure or with the ECG?
a. Triggering Decision: The decision to trigger the IABP with either pressure or ECG signals depends on the patient’s specific circumstances and the clinical team’s preference.
b. ECG Triggering: ECG-triggered counterpulsation is generally preferred, providing more precise timing and synchronization with the cardiac cycle. This ensures that the balloon inflates during diastole and deflates during systole, maximizing the hemodynamic benefits.
c. Pressure Triggering: Pressure-triggered counterpulsation may be used when ECG signals are unreliable or unavailable, but it can lead to suboptimal timing and reduced hemodynamic benefits. Pressure-based triggering relies on arterial pressure waveforms, which can be influenced by various factors, potentially leading to inaccurate timing.
To Sum it up:
The simultaneous use of an IABP with Bi-Fem Peripheral VA ECMO is a complex and challenging endeavor that requires a thorough understanding of the underlying principles, potential complications, and careful monitoring. Learning about the combination of these technologies offers invaluable insights into integrated patient management for someone like me. The coordination needed to balance these therapies provides a deep understanding of advanced hemodynamics, device interplay, and personalized patient care strategies. As an ECMO Specialist, you must be committed to continuous education, and staying up-to-date with the latest research, guidelines, and best practices is crucial. It inspires us to ensure safe and effective interventions. Continuous education, multidisciplinary collaboration, and a patient-centered approach are vital to navigating these intricate scenarios and providing the best care for our critically ill patients.
Please leave a comment if you have something to add, and hit the Subscribe button below to receive notifications of new posts.
Note: This article reflects my learning journey in ECMO and is intended for educational purposes only. It should not be used as a substitute for professional medical advice or guidance. Always consult with qualified healthcare professionals for clinical decisions and patient care.
Acknowledgments
I developed two custom GPTs, "AI ECMO Expert" and "ECMO Specialist Handover Practice," for specialized research. These tools draw primarily from the ELSO Redbook (6th Edition), the ELSO Specialist Training Manual (4th Edition), various research papers, and articles. Additional research was supported by GPT-4o, Claude 3 Sonnet, and Perplexity. Editing was performed with Grammarly, and AI visuals were created using Leonardo AI and DALL-E3 AI Image Generator.